Clinical Research
Mabpharm Limited, a pioneering biopharmaceutical company, specializes in the R&D and manufacturing of novel drugs and biosimilars targeting cancer and autoimmune conditions. The company boasts an extensive portfolio of antibody therapeutics, including several monoclonal antibody drugs in its pipeline. Some of these drugs are already in clinical trials and have the potential to provide innovative treatment alternatives to a broader patient population in the coming years.
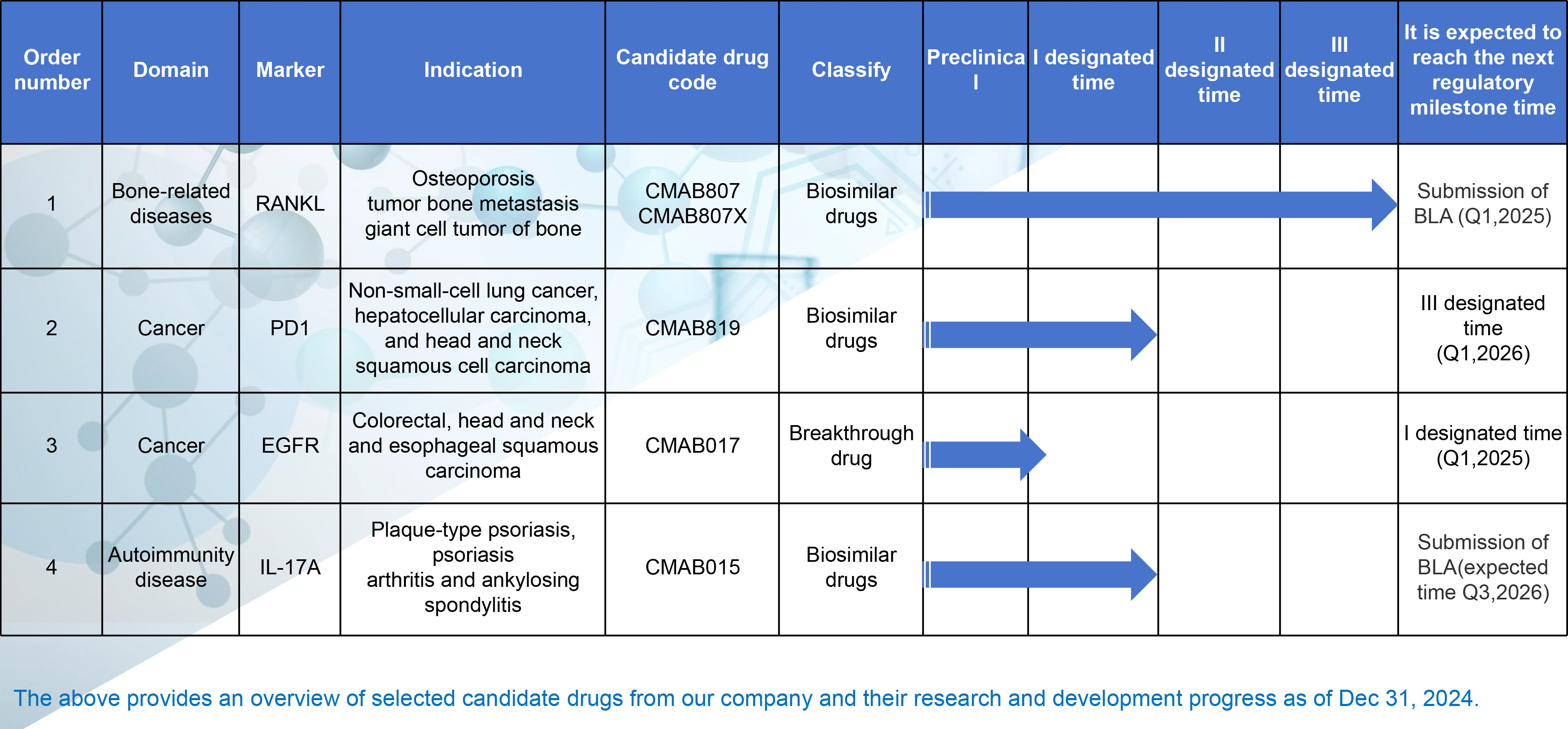
While strengthening the manufacturing of existing marketed drugs, our company continues to invest in R&D, promoting the discovery and launch of new drugs to address potential drug shortages and emerging disease challenges. Mabpharm Limited is providing more treatment options and offering strong support for public health security through its rich reserve of clinical drugs.
A human immunoglobulin G2 (IgG2) monoclonal antibody with affinity and specificity for human RANKL (Receptor Activator of Nuclear Factor-κB Ligand), which is a transmembrane or soluble protein essential for the formation, function, and survival of osteoclasts (cells responsible for bone resorption). CMAB807/CMAB807X prevents RANKL from activating the receptor RANK on the surface of osteoclasts and their precursors. By blocking the interaction between RANKL and RANK, it inhibits the formation, function, and survival of osteoclasts, thereby reducing bone resorption and increasing the bone mass and strength of cortical and trabecular bone.
CMAB015 is a biosimilar candidate for Cosentyx® (Secukinumab), which is a fully human monoclonal IgG1 antibody. Its primary mechanism of action is to selectively bind to and inhibit interleukin-17A (IL-17A), a key factor in the inflammatory pathway, preventing its binding to the interleukin-17 (IL-17) receptor and thereby reducing inflammatory responses. The indications for this drug include moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis.
As a novel probody drug, CMAB017 features a design of blocking peptides that are expected to significantly reduce adverse reactions such as skin and gastrointestinal mucosa reactions. The selection of the human immunoglobulin G1 (IgG1) constant region can enhance the antibody's Fc-mediated effects, thereby improving therapeutic efficacy. Compared with similar products already on the market, CMAB017 is a first-in-class biological drug with improved efficacy and safety. Furthermore, it is anticipated that additional probody drugs will be developed using the CMAB017 R&D platform. CMAB017 is indicated for the treatment of advanced solid tumors, including but not limited to colorectal cancer, squamous cell carcinoma of the head and neck, and esophageal squamous cell carcinoma.
CMAB819 is a biosimilar drug candidate, and the National Medical Products Administration (NMPA) has approved its clinical trials. We have completed Phase I clinical trials and expect CMAB819 to obtain marketing approval from the NMPA in the fourth quarter of 2028. CMAB819 is indicated for the treatment of metastatic non-small cell lung cancer, hepatocellular carcinoma, and squamous cell carcinoma of the head and neck.


